Prion diseases, or transmissible spongiform encephalopathies (TSEs), are a group of rare, incurable neurodegenerative disorders leading to motor and cognitive impairments, extensive brain damage and neuronal degeneration. These diseases include Creutzfeldt-Jakob disease (CJD), Gerstmann-Straussler-Scheinker Syndrome (GSS), Fatal Familial Insomnia (FFI) and kuru in humans; scrapie in sheep and goats, bovine spongiform encephalopathy (BSE) in cattle, and chronic wasting disease (CWD) in cervids.
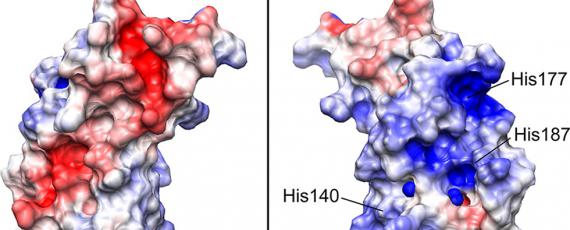
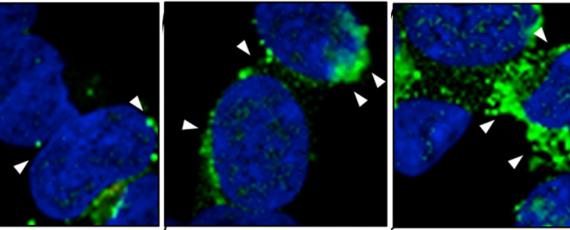
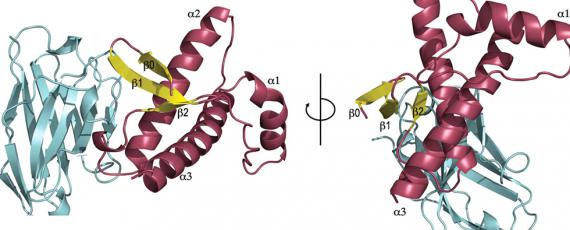
These diseases have considerable interest as they represent a new biological principle of infection, where the disease agent, unlike other pathogens, is composed solely or principally of a protein, specifically the prion protein. The prion, denoted PrPSc for scrapie prion protein, is the abnormal, prion disease-causing form of the physiologically expressed cellular prion protein, or PrPC. Conversion of PrPC into the abnormally folded, β-sheet enriched and protease resistant isoform, PrPSc, is the hallmark of prion diseases. PrPSc is prone to accumulate and aggregate in the brain of affected individuals leading to neurodegeneration.
Prion diseases can be divided into three etiological categories: sporadic, inherited, and acquired. Importantly, even the sporadic and genetic forms of the disease are experimentally transmissible.
Prion diseases, while rare, have gained more attention as a growing number of scientific studies have suggested that the prion mechanism of neurodegenerative pathology may not be limited to prion diseases. Many neurodegenerative diseases that involve misfolded and aggregated proteins, such as Alzheimer’s disease (AD) and Parkinson’s disease (PD), may share with prion diseases analogous mechanisms of conversion, replication and propagation. For this and much more, self-propagating proteins such as prions continue to be a source of scientific interest.